In the preclinical phase, formulation development is usually very standardized, limited in time and follows heuristic guidelines. The digital design approach to drug formulation developed by amofor offers a unique alternative for customers looking for more efficient approaches. This innovative method enables the development of highly potent preclinical formulations in just one day, allowing a faster transition to first-in-human studies.
Achieving High Bioavailability for Preclinical Toxicology Testing
The main goal of preclinical toxicology testing is to formulate active pharmaceutical ingredients (APIs) with enormous bioavailability. The tested molecules must enter the bloodstream properly so that we find out whether these molecules show harmful side effects and at what dose these occur. High doses of the API need to be absorbed by the body to effectively test the safety also at too high concentrations. A major challenge is to prevent the active pharmaceutical ingredient (API) from simply passing through the body without having the intended bioavailability. A bunch of different platform techniques is potentially available for this purpose, it is only necessary to pick the right one based on the physicochemical properties. This highlights the need for a robust formulation platform that ensures a good release profile, maximizes exposure of the drug in animal models and accurately verifies its safety.
Formulation Constraints in Preclinical Tox-Studies
Preclinical toxicological studies typically apply liquid, subcutaneous formulations, which need to maintain physical stability only for a certain time – for a few days up to a week – far less than the three-year stability requirement for clinical trial drugs. This abbreviated stability period in a completely different environment required completely different approaches than in later formulation development. But, given the constraints of time and the need for high drug exposure, figuring out the best mix can feel like solving a puzzle. What is the best vehicle for the formulation? Should I add a co-solvent, even if it might be harmful? Is it beneficial to add a specific polymer to stabilize my API in this aqueous slurry?
Digital Drug Formulation Helps
amofor addresses the challenges in early drug formulation by using a comprehensive digital drug formulation methodology. Through our in-silico-based systematic decision tree (see below), we can rapidly evaluate different excipients and their interactions with the API to identify the most effective combinations for high bioavailability. It speeds up the process of deciding on co-solvents and reduces the number of tedious and conventional experimental methods of optimization and development.
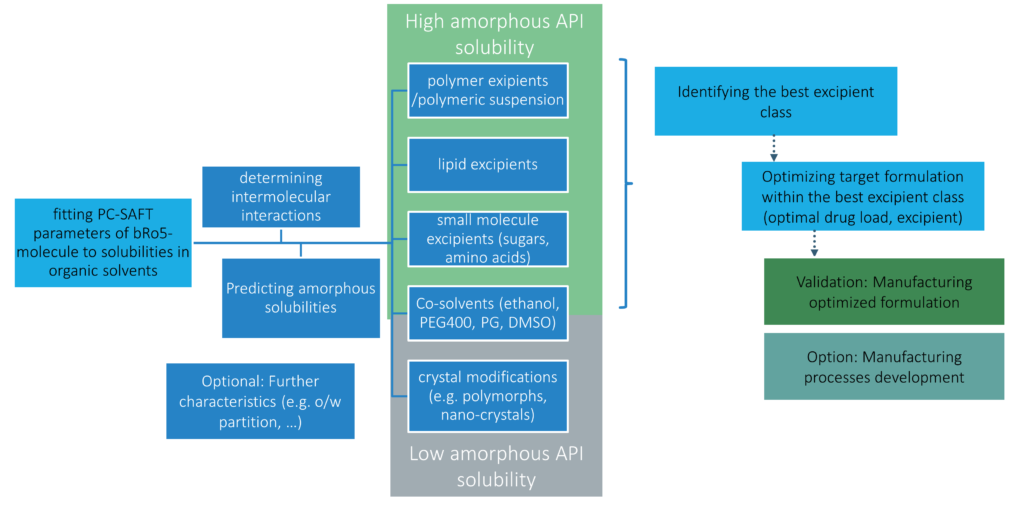
amofor’s rapid formulation method streamlines the drug development process, offering several advantages. Not only does it save material, but it also enables researchers to quickly begin important studies. We can make clear recommendations for effective formulations, eliminating the need for extensive preliminary testing. This efficiency makes it possible to focus directly on the most important areas of research.
Key Benefits:
- Rapid Prototyping: Achieve ready-to-test formulations within a single day.
- High Bioavailability: Guarantees thorough evaluation of efficacy and safety.
- Cost Efficiency: Significantly lowers both financial and resource costs in preclinical trials.
- Precision and Accuracy: Delivers formulations precisely tailored to meet specific research needs and tailored to the individual intermolecular interaction profile.
Key Takeaways
The early stages of drug development are crucial but often filled with uncertainties. At this early stage, much about the molecule is still enigmatic and many aspects have yet to be clarified or fully understood. For this reason, an in silico modeling approach can play a crucial role right from the start.
Entering in the digital design of drug formulations as early as possible offers a strategic advantage. It helps to navigate the unknowns and is not limited to a single development phase, but encompasses the entire spectrum, from the first to the last stages of drug development. Once the foundation is laid, it paves the way for a seamless transition and continuity in the subsequent phases of formulation development.
Recommended review to dive deeper into the topic of early formulations: Developing early formulations: Practice and perspective
If you have any questions about our modeling approach, please contact us. Thank you!