“It’s a job you can’t find anywhere else.” That’s how Marius Rother, not without enthusiasm, describes his highly specialized role at amofor. It’s a position at the intersection of science, software development, and tailored problem-solving in drug formulation. With these simulations, Marius helps pharmaceutical companies tackle their toughest formulation challenges using physics-based simulation tools.
But what is it really like to work at amofor? In this interview, Marius shares the problems he solves, how he does it, and why he decided to join amofor.
Can You Give An Example Of Customer Projects You Have Supported Already?
A common challenge we see is when a client has a formulation that performs poorly, but the root cause is a complete mystery. The composition looks right, but the results are wrong. This is where modeling provides a unique advantage.
In one instance, a customer came to us with a familiar but frustrating situation: Two drug formulations. Nearly identical on paper. One performed as intended in vivo. The other did not.
Standard analytical methods showed no meaningful differences. Both systems should have behaved the same. Instead of adding another round of trial-and-error experiments, we went back to first principles.
We developed two complementary, physics-based models:
– one describing molecular transport and release behavior
– another capturing a subtle, often overlooked interfacial property that governs how long a formulation can remain functionally effective at the site of action
Our analysis led to a clear, mechanistic hypothesis:
The underperforming prototype was losing this critical functional interaction too early in the process. Targeted mechanical experiments later confirmed the hypothesis in vitro.
Building on this, we combined, release kinetics, molecular mobility and this interfacial performance descriptor into a single, robust indicator that correlates with in vivo outcome.
The key insight was not simply which formulation worked better. It was WHY it worked better: because it preserved its functional performance during a decisive time window, and because the underlying mechanism could be quantified and predicted. This kind of modeling allows formulation teams to move beyond empirical screening and base decisions on mechanisms that are normally inaccessible to standard analytics.
The project is a great example of why I enjoy this work. We didn’t just deliver numbers; we diagnosed the root cause. It also shows our ability to approach problems creatively, often building new, and first principle models tailored to the specific challenge.
What Role Does Software Play in Your Work at amofor?
Most of my work involves expanding the software’s capabilities and pushing it into new areas.
Typically, our modeling begins by combining different sources of input, a-priori calculations, our in-house data bank, and PC-SAFT parameters. These foundational elements feed into SOLCALC, our thermodynamic modeling platform, and power the simulations we use to analyze phase behavior, solubility, and formulation performance across complex systems.
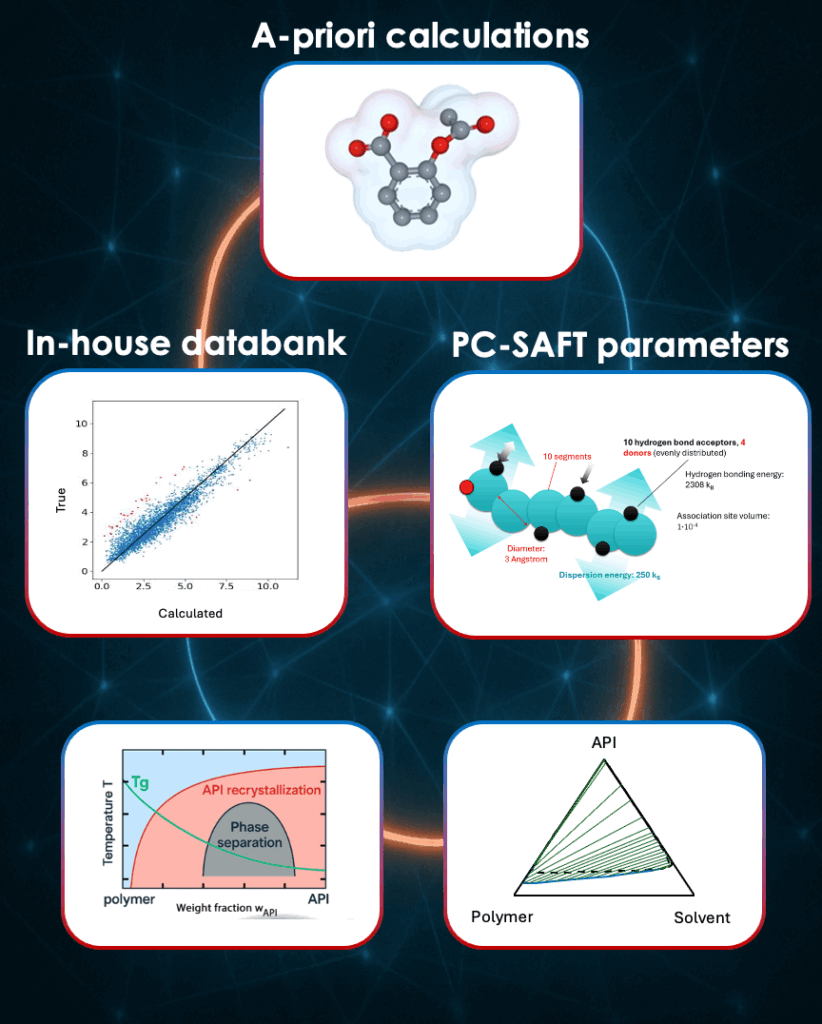
How we build predictive formulation models: A-priori calculations, experimental data, and PC-SAFT parameters form the
foundation of our simulations.
A key challenge in using PC-SAFT – the equation of state behind SOLCALC – is parameter estimation. To model a new compound, we need five solubility measurements. We already have a big data framework that helps us make educated guesses for new components using existing solubility data. But that’s not always possible, especially when the compound only exists virtually (usually the case at earliest stages of development). That’s why we want to improve this by integrating parameters based on molecular structure directly, bridging the gap between database-driven modeling and predictive, physics-based first principles. The goal is to reduce the number of lab measurements needed and still get the best possible simulation result.
And of course, whenever we implement a new method, we also have to validate it thoroughly. That’s often the most time-consuming part, making sure the model works across different systems and delivers reliable results.
Another big focus is improving user experience of the software. Right now, SOLCALC is a powerful software, but users still need understanding of thermodynamics. We want to simplify that. We’re building more guided workflows and cleaner graphical interfaces so users can’t make critical input mistakes. Think of it like moving from a raw toolbox to a GPS-guided interface: we guide them step by step, reducing the chance of errors and making the software more accessible, especially for formulators without a modeling background.
Why Did You Join amofor?
It started back in 2020 during my bachelor’s thesis. Christian Lübbert was supervising it, and toward the end, he asked if I’d be interested in helping with the development of modeling tools.
At first, I worked part-time during my master’s and PhD, mostly handling maintenance tasks for SOLCALC. But over time, I got a deeper look at how Christian worked with companies. One drug formulator had questions about drug solubility, another about stability, and every project combined different concepts from our studies in a new way. That variety was the hook.
Since April 2025, I’ve been working full-time. I directly apply what I’ve learned, grow technically, and work with people from all over the world. The cutting-edge and first principle models models we’re developing, combined with the chance to solve real formulation problems, is a big part of the appeal.
What’s A Common Misconception About In-Silico Modeling?
That the simulations we run are always correct. But every model and simulation comes with uncertainty. The key is understanding how wrong a result might be, and whether that level of error is acceptable for the decision you’re trying to make. We always try to communicate that clearly. It’s not about getting the exact number. It’s about understanding the trend, the risk, and the underlying mechanism.
What Does It Take To Work At amofor?
It’s a job you cannot find anywhere else because it’s so hyper-specialized. You need a mix of skills and knowledge: thermodynamics, numerical mathematics, physics, coding, and a solid understanding of pharmaceutical science.
One example: I’ve worked on simulating thermal cycling experiments, heating and cooling a sample to identify phase transitions like melting or recrystallization. These simulations help us extract properties like melting entropy or melting temperature. The theory behind this goes back to the 1940s, to researchers like Melvin Avrami [1], but the math is complex. You have to solve a system of differential equations, often implicitly. That requires building custom numerical solvers and implementing algorithms that produce accurate, reliable results.
And it’s not just about mathematics. A mathematician might calculate a solution, but without physical context, they can’t judge if it makes sense. You need both perspectives: mathematical rigor and physical insight.You’re constantly solving problems that no one has solved before, which demands both depth and breadth. That’s not easy, but it’s what makes the job so interesting and rewarding.
What Excites You About The Future Of Formulation?
For the last 50 years, drug formulation relied on trial and error, mixing components, testing, and seeing what happens.
But now, formulation is finally moving into the digital age. We can simulate steps to make lab work more focused. Instead of testing 100 samples, you test 10, and you understand why those 10 are critical. Of course, there’s still a lot of work to do, especially around validation and building confidence in the simulations.
We’re also starting to explore what’s possible with machine learning and quantum computing. The better we get at predicting behavior from molecular structure, the earlier we can make smart decisions in the development process. Of course, building confidence in simulations is key. But I believe we’re just at the beginning of a big shift. Seeing how much more we’ll be able to do in the coming years is incredibly exciting.
—
Whether you need deeper insight into a formulation issue or want to accelerate development, amofor’s team is here to help.
Contact us to schedule a consultation or demo.
[1] M. Avrami, Kinetics of Phase Change. I General Theory, J. Chem. Phys. 7, 1939, DOI: https://doi.org/10.1063/1.1750380


